Eye ointment used for dry eyes recalled over risk of infection, blindness
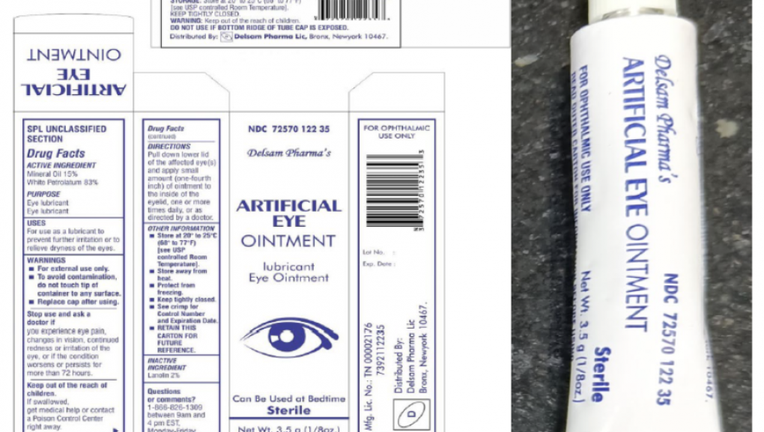
The Batch No. H29 of Artificial Eye Ointment, distributed by Delsam Pharma, is pictured in a provided photo. (Credit: FDA / Global Pharma Healthcare)
WASHINGTON - A company that previously recalled over-the-counter eye drops linked to an outbreak of drug-resistant infections recently expanded the notice to include eye ointment cream.
The U.S. Food and Drug Administration announced the expanded recall of Global Pharma Healthcare’s Artificial Eye Ointment – distributed under the brand name Delsam Pharma – due to possible microbial contamination.
Using certain batches of the recalled product could result in an eye infection "that could lead to blindness," the FDA said in a Feb. 24 notice.
The agency added that some packaging of the recalled product is also leaking or may otherwise be compromised.
Recalled Artificial Eye Ointment: What to know
The Artificial Eye Ointment (mineral oil 15%, white petrolatum 83%, 3.5 grams / 1/8 oz.) is used as an eye lubricant and to relieve dryness, the FDA notice states.
The recalled product is Batch No. H29 and is packaged in a white aluminum tube within a paper carton. It was distributed nationwide in the U.S., and by Delsam online.
Global Pharma Healthcare notified Delsam Pharma about the recall and was requesting that wholesalers, retailers, and customers who have the impacted product to stop any use and discard it, according to the FDA.
Delsam Pharma’s NDC for this product is 72570-122-35, and its UPC code is 3 72570 012235 3.
Anyone who has experienced issues that may be related to the product was urged to contact their doctor and report it to the FDA.
Global Pharma Healthcare said it had not received any reports of adverse events related to the product to date.
Previous eye drop recall linked to death, multi-state bacterial infections
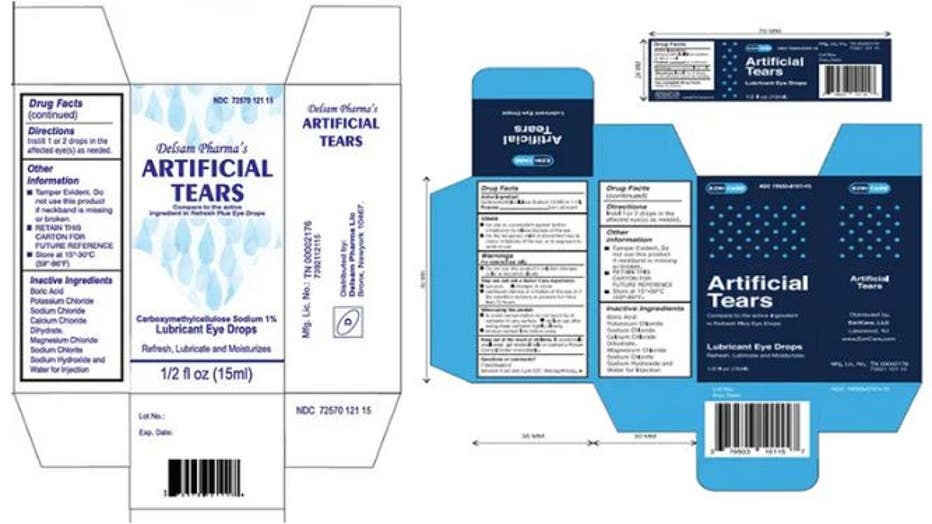
Global Pharma Healthcare is voluntarily recalling all lots within the expiry of their Artificial Tears Lubricant Eye Drops, distributed by /EzriCare, LLC- and Delsam Pharma, due to possible contamination. (Credit: FDA)
On Feb. 2, Global Pharma also recalled all unexpired lots of EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears after being linked to a multi-state outbreak of bacterial infections.
The U.S. Centers for Disease Control and Prevention had previously sent a health alert to doctors, saying the outbreak included at least 55 people in 12 states. One died and at least five others had permanent vision loss.
The infections, including some found in blood, urine and lungs, were linked to EzriCare Artificial Tears. Many said they had used the product, which is a lubricant used to treat irritation and dryness.
The eye drops are sold under the name EzriCare and are made in India by Global Pharma Healthcare. The FDA recommended the recall based on manufacturing problems including a lack of testing and proper controls on packaging.
The agency also blocked imports into the United States.
The infections were caused by a bacteria called Pseudomonas aeruginosa. Investigators detected it in open EzriCare bottles, but further testing was underway.
EzriCare, the company that markets the eye drops in the U.S., said it is not aware of any evidence definitively linking the outbreak to the product, but that it had stopped distributing the eye drops. It also has a notice on its website urging consumers to stop using the product.
Infections were diagnosed in patients in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington and Wisconsin. A person in Washington died with a blood infection.
The outbreak is considered particularly worrisome because the bacteria driving it are resistant to standard antibiotics, officials said.
This story was reported from Cincinnati. The Associated Press contributed.

